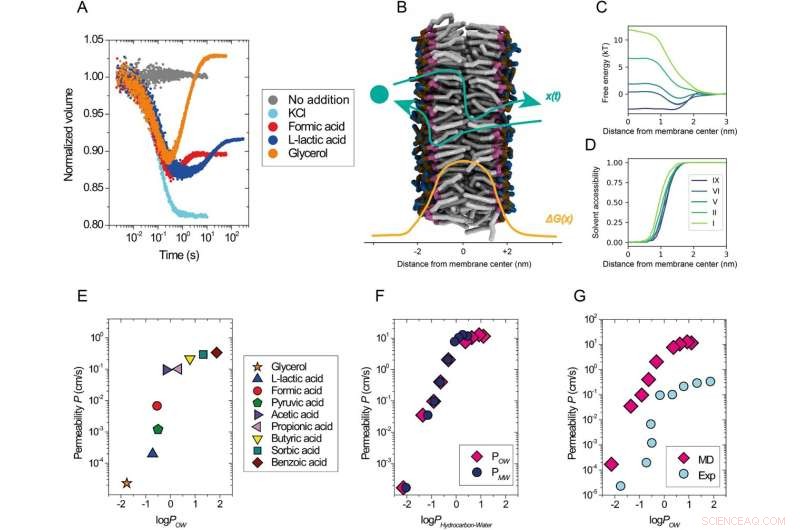
Permeabilità dei soluti in funzione della loro lipofilia. Una panoramica del saggio sperimentale. Dati cinetici ottenuti con il test di auto spegnimento della calceina utilizzando vescicole composte da DOPC miscelato con tampone (grigio) o scioccate osmoticamente con KCl 52,5 mM (ciano), formiato di sodio 50 mM (rosso), L-lattato di sodio 50 mM (blu) o 120 mM glicerolo (arancione) a 20 °C. B Descrizione schematica del processo di permeazione, x(t), attraverso una membrana lipidica con un profilo energetico libero di esempio, ΔG(x) (code lipidiche, grigio; porzione glicerolo, viola; porzione fosfato, ocra; e porzione colina, blu; le molecole d'acqua non sono mostrate). C Profili di energia libera selezionati da simulazioni di soluti con livelli di idrofobicità variabili (I più idrofili, IX più idrofobici) che permeano attraverso una membrana lipidica DOPC in funzione della distanza dal centro del doppio strato lungo la membrana. Viene mostrata solo la metà dell'intero profilo di permeazione simmetrica. D Profili di accessibilità ai solventi dei soluti permeanti lungo il percorso di permeazione. I soluti interagiscono con le molecole di solvente anche in profondità nella regione della coda della membrana (x < 1,0 nm). E–G Coefficienti di permeabilità per membrane DOPC da esperimenti a 20 °C (E, G) e simulazioni (F, G) tracciati rispetto al logaritmo di ottanolo/acqua (logPOW ) e coefficiente di ripartizione membrana/acqua (logPMW ) dei soluti. Soluti dei livelli idrofobici I (logPOW = −2.14) a IX (logPOW = 1,1) sono stati utilizzati nelle simulazioni. AccediP è log10 (coefficiente di partizione), dove P si riferisce alla distribuzione di equilibrio di una molecola tra la fase idrofobica e idrofila di due solventi immiscibili. Il log sperimentalePOW i valori per acidi deboli e glicerolo (E, G) sono stati presi dal database PubChem (https://pubchem.ncbi.nlm.nih.gov/). Il log simulatoPOW i valori (F, G) sono stati presi dal lavoro25; logPMW i valori (F) sono stati ottenuti dalle presenti simulazioni. I coefficienti di partizione sono relativi alla differenza di energia libera tra le rispettive fasi. Nel caso di PMW , questa è la differenza tra il centro della membrana e la fase solvente. I valori dell'energia libera sono presentati in (C). Credito:Comunicazioni sulla natura (2022). DOI:10.1038/s41467-022-29272-x
Batteri, funghi e lieviti sono molto bravi a espellere sostanze utili come gli acidi deboli. One way in which they do this is through passive diffusion of molecules across the cell membrane. At the same time, cells need to prevent leakage of numerous small molecules. Yeast cells, for instance, can live in hostile environments thanks to a very robust and relatively impermeable membrane system. Biochemists at the University of Groningen have studied how the composition of the membrane affects passive diffusion and the robustness of the cell membrane. Their results, which were published in Nature Communications on March 25, could help the biotech industry to optimize microbial production of useful molecules and help in drug design.
Border control is very important to cells. Their membranes separate the inner and outer environments, which are quite different. To absorb useful compounds, such as nutrients, or to excrete waste, cells can use selective transport systems. However, some transport across the membrane takes place by passive diffusion. This is a non-selective process that will let some molecules go in or out, depending on their size and hydrophobicity, for example. Active transporters have been studied extensively; however, our knowledge of passive diffusion through the membrane is still very incomplete.
Synthetic vesicles
This is a problem for the biotechnology industry, which uses cells as factories to produce a myriad of useful substances and that needs these worker cells to survive under harsh conditions, for example in an environment with a high alcohol or weak acid concentration. Bert Poolman, Professor of Biochemistry at the University of Groningen, was approached by a biotech company that was interested in producing lactic acid in bacteria. They wanted to know more about passive diffusion. This fitted in nicely with another project that Poolman is working on. "We are highly interested in these passive transport processes because of our involvement in a project to build a synthetic cell," says Poolman. "If you can use passive diffusion instead of an active transport system, you need fewer parts to construct such a cell."
So, he combined both questions in a research project. "We started out with a systematic study of what causes the differences in permeability of yeast membranes and bacterial membranes," says Poolman. His team created synthetic vesicles that were made up of three to four different lipids. Ergosterol or cholesterol was added to the membranes to affect their fluidity and rigidity. A range of small molecules was tested using this system and the results from these experiments guided molecular dynamic simulations of diffusion through membranes. The in-silico studies, supervised by Professor Siewert-Jan Marrink, provided a deeper insight into the molecular mechanism of diffusion.
Tweaking
The fatty acid tails of the lipids turned out to be most important in determining the properties of membranes, whereas the hydrophilic head groups had little effect on the permeability. The length of the tails also mattered. "And saturated tails, with no double carbon bonds, are stiffer than unsaturated ones. Hydrophobic interactions cause a close packing of these tails, resulting in a gel phase that is not very penetrable," explains Poolman. Sterols increase the fluidity but in the case of yeast, which uses ergosterol, the permeability remains low. "Thus, by tweaking the saturation of the fatty acids and the type and amount of sterol in the membrane, we can modify the permeability of the plasma membrane of yeast and bacterial cells."
Poolman and his colleagues have, therefore, defined a number of variables that alter the permeability of membranes for different classes of compounds. This information can be used by companies that use yeasts or bacteria as cell factories. "However, our results cannot be directly applied to those cells," says Poolman. "Real membranes contain hundreds of different lipids and the composition can vary between different locations in the membrane. In addition, these cell membranes contain all kinds of proteins. If you make changes in, for example, the lipid composition of the membrane, a lot can go wrong and the function of a membrane protein can be affected."
Drug design
The increased understanding of the physical processes that affect permeability can help companies to understand why certain cells are better for specific processes than others. "The usual way to tweak strains is by directed evolution. Our results will help companies to better understand the results of those optimizations and guide their cell engineering efforts."
Another application is the design of drugs that act inside cells. "Pharmaceutical companies use a set of empirically established rules to optimize drugs for action inside cells, based on parameters such as size or polarity. Our study highlights the importance of the membrane composition of the targeted cells and this could help in drug design."